Are you curious about the world of pharmaceutical research and development? Have you ever wondered who takes care of the intricate details of drug trials and clinical studies? Look no further, as we delve into the fascinating realm of Contract Research Organizations (CROs). In this comprehensive guide, we will explore what CROs are, their significance, services offered, and the impact they have on the pharmaceutical industry.
About CROs
CROs, or Contract Research Organizations, are specialized entities that provide research and development services to pharmaceutical companies, biotech firms, and medical device manufacturers. These organizations act as external partners, handling various aspects of drug development, from early-stage research to clinical trials and post-market surveillance.
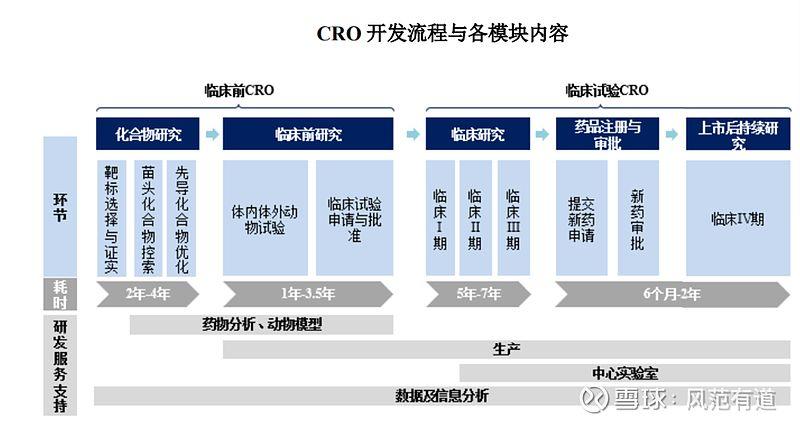
Originating in the early 1980s, CROs have become an integral part of the pharmaceutical industry. They offer a range of benefits, including increased efficiency, reduced costs, and access to specialized expertise. By outsourcing certain tasks to CROs, pharmaceutical companies can focus on their core competencies and accelerate the drug development process.
Services Offered by CROs
CROs provide a wide array of services to support drug development. Here are some of the key services they offer:
| Service | Description |
|---|---|
| Drug Discovery | Identifying and optimizing potential drug candidates through various screening techniques. |
| Preclinical Studies | Conducting studies in laboratory animals to evaluate the safety and efficacy of drug candidates. |
| Phase I Clinical Trials | Testing the safety and pharmacokinetics of a drug in a small group of healthy volunteers. |
| Phase II Clinical Trials | Evaluating the efficacy and safety of a drug in a larger group of patients. |
| Phase III Clinical Trials | Confirming the efficacy and safety of a drug in a large, diverse population. |
| Post-Marketing Surveillance | Monitoring the safety and effectiveness of a drug after it has been approved for use. |
Additionally, CROs offer services such as data management, statistical analysis, regulatory affairs, and medical writing. This comprehensive suite of services allows pharmaceutical companies to streamline their drug development process and bring new medications to market more quickly.
The Importance of CROs in Drug Development
CROs play a crucial role in the drug development process for several reasons:

-
Expertise: CROs have specialized knowledge and experience in various aspects of drug development, allowing them to provide high-quality services.
-
Cost-Effectiveness: Outsourcing certain tasks to CROs can reduce costs for pharmaceutical companies, as they can avoid investing in expensive equipment and hiring additional staff.
-
Efficiency: CROs are dedicated to drug development and can complete tasks more quickly than internal teams, allowing for faster time-to-market.
-
Regulatory Compliance: CROs are well-versed in regulatory requirements and can help ensure that drug development processes are compliant with applicable regulations.
Impact on the Pharmaceutical Industry
The rise of CROs has had a significant impact on the pharmaceutical industry. By providing specialized services, CROs have helped to accelerate the drug development process, leading to more new medications being approved and brought to market. This has, in turn, improved patient outcomes and increased access to innovative treatments.
Moreover, CROs have facilitated collaboration between pharmaceutical companies, biotech firms, and academic institutions, fostering innovation and knowledge sharing. This collaboration has led to the development of new drug delivery systems, combination therapies, and personalized medicine approaches.
Conclusion
CROs are an essential component of the pharmaceutical industry, providing specialized services that help accelerate drug development and improve patient outcomes. As the industry continues to evolve, CROs will undoubtedly play an increasingly important role in bringing new medications to market.