+oncology +cro: A Comprehensive Overview
When it comes to the field of oncology, collaboration with Contract Research Organizations (CROs) has become an integral part of the drug development process. This article delves into the multifaceted relationship between oncology and CROs, exploring the benefits, challenges, and key considerations for successful partnerships.
Understanding Oncology
Oncology, the branch of medicine that deals with the prevention, diagnosis, and treatment of cancer, has seen significant advancements in recent years. With the rise of personalized medicine and targeted therapies, oncologists are now able to tailor treatments to individual patients, leading to better outcomes and improved quality of life.
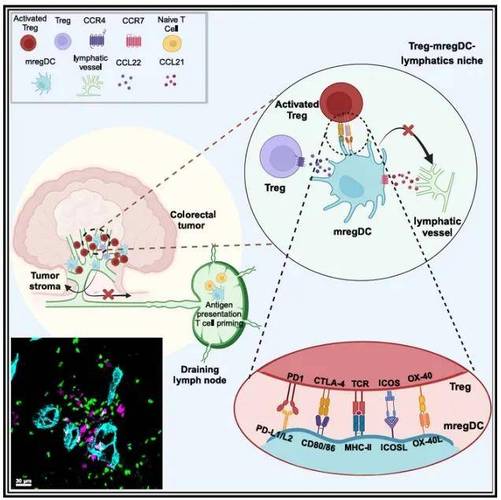
The Role of CROs in Oncology
CROs play a crucial role in the oncology drug development process by providing specialized services that help pharmaceutical companies bring new treatments to market. These services include clinical trials management, data management, biostatistics, and regulatory affairs.
Benefits of Collaborating with CROs
Collaborating with CROs offers several benefits to oncology researchers and pharmaceutical companies. Here are some of the key advantages:
-
Expertise: CROs have extensive experience in conducting clinical trials and managing drug development processes, which can help ensure the success of oncology studies.
-
Cost-Effectiveness: Outsourcing certain aspects of drug development to CROs can help reduce costs and streamline the process.
-
Speed: CROs are often able to complete studies more quickly than internal teams, allowing for faster approval of new treatments.
-
Regulatory Compliance: CROs are well-versed in regulatory requirements and can help ensure that oncology studies meet all necessary standards.
Challenges of Collaborating with CROs
While collaborating with CROs offers numerous benefits, there are also challenges to consider:
-
Communication: Effective communication between oncology researchers and CROs is essential for the success of a partnership. Language barriers and cultural differences can sometimes hinder communication.
-
Data Security: Ensuring the confidentiality and security of patient data is a critical concern when working with CROs.
-
Quality Control: Maintaining high standards of quality control throughout the drug development process is essential, and this can be challenging when working with external partners.
Key Considerations for Successful Partnerships
To ensure a successful partnership between oncology researchers and CROs, it is important to consider the following factors:
-
Selection of CRO: Choose a CRO with a strong track record in oncology and a reputation for quality and reliability.
-
Clear Communication: Establish clear lines of communication and maintain regular updates throughout the partnership.
-
Data Sharing: Develop a robust data-sharing agreement that ensures the confidentiality and security of patient data.
-
Quality Control: Implement a comprehensive quality control process to ensure the integrity of the data and the study outcomes.
Case Studies: Successful Oncology-CRO Collaborations
Several notable oncology studies have been successfully conducted through collaborations with CROs. Here are a few examples:
