CRO DF: A Comprehensive Guide to Understanding Data in Clinical Research Organizations
As a key component in the world of clinical research organizations (CROs), the term “CRO DF” refers to a data framework that is integral to the management and analysis of clinical trial data. In this detailed guide, we will delve into what CRO DF entails, its significance, and how it impacts the efficiency and accuracy of clinical trials.
What is CRO DF?
CRO DF, or Data Framework, is a structured and standardized approach to organizing, storing, and managing clinical trial data. It serves as a foundation for data integrity, accessibility, and analysis within a CRO. The framework typically includes various components such as data dictionaries, data models, and data standards.
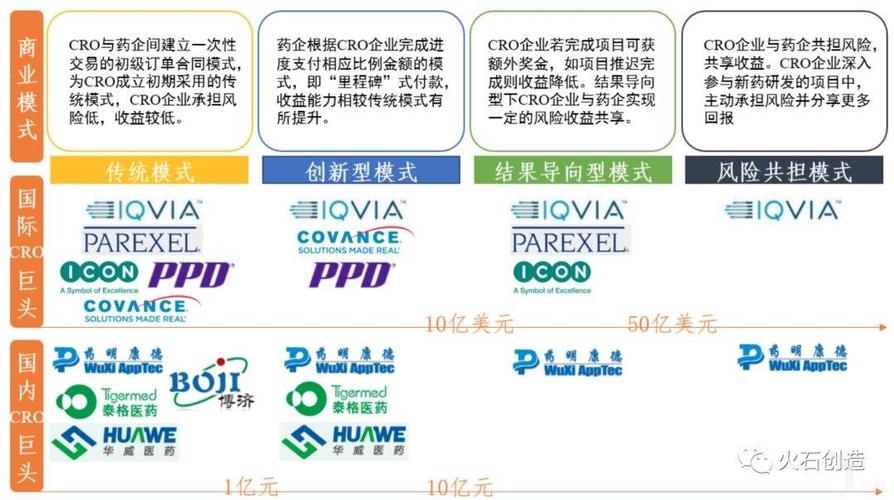
Components of CRO DF
Let’s take a closer look at the key components that make up a CRO DF:
| Component | Description |
|---|---|
| Data Dictionaries | Define the meaning, format, and usage of data elements within the clinical trial. |
| Data Models | Represent the structure and relationships between data elements. |
| Data Standards | Ensure consistency and interoperability of data across different systems and platforms. |
Significance of CRO DF
The significance of CRO DF cannot be overstated, as it plays a crucial role in the following aspects:
-
Enhanced Data Quality: By adhering to standardized data frameworks, CROs can ensure the accuracy, consistency, and reliability of clinical trial data.
-
Improved Data Accessibility: CRO DF facilitates easy access to data for various stakeholders, including researchers, regulatory authorities, and patients.

-
Efficient Data Analysis: With a well-defined CRO DF, data analysis becomes more streamlined, enabling faster insights and decision-making.
-
Regulatory Compliance: CRO DF helps ensure compliance with regulatory requirements, such as the Good Clinical Practice (GCP) guidelines.
Impact on Clinical Trials
The implementation of CRO DF has a direct impact on the success of clinical trials. Here are some key aspects:
-
Reduced Data Errors: Standardized data frameworks minimize the risk of data errors, leading to more reliable results.
-
Increased Efficiency: Streamlined data management processes save time and resources, allowing CROs to conduct more trials in less time.
-
Enhanced Collaboration: CRO DF fosters better collaboration between researchers, clinicians, and regulatory authorities.
-
Improved Patient Outcomes: By ensuring the quality and accuracy of clinical trial data, CRO DF contributes to the development of effective treatments and better patient outcomes.
Challenges and Best Practices
While CRO DF offers numerous benefits, implementing it can come with its own set of challenges. Here are some common challenges and best practices to overcome them:
-
Challenge: Data Integration
Best Practice: Invest in robust data integration tools and establish clear data governance policies.
-
Challenge: Data Security
Best Practice: Implement strict data security measures, including encryption and access controls.
-
Challenge: Data Quality
Best Practice: Regularly audit and validate data to ensure its accuracy and reliability.
Conclusion
CRO DF is a vital component in the world of clinical research organizations. By implementing a well-defined data framework, CROs can enhance data quality, improve efficiency, and contribute to the development of effective treatments. As the clinical research landscape continues to evolve, embracing CRO DF will be crucial for CROs to stay competitive and deliver successful clinical trials.
