Animal Studies Preclinical Cro: A Comprehensive Overview
Animal studies play a pivotal role in the preclinical phase of drug development. This article delves into the intricacies of animal studies in preclinical cro, offering a detailed and multi-dimensional perspective. By exploring various aspects, we aim to provide a comprehensive understanding of this critical phase in the pharmaceutical industry.
Understanding Preclinical Cro
Preclinical cro refers to the initial phase of drug development, where researchers investigate the potential efficacy and safety of a drug candidate. This phase involves extensive testing on animals before moving on to human trials. Animal studies are crucial in this phase as they help identify the drug’s pharmacokinetics, pharmacodynamics, and potential side effects.
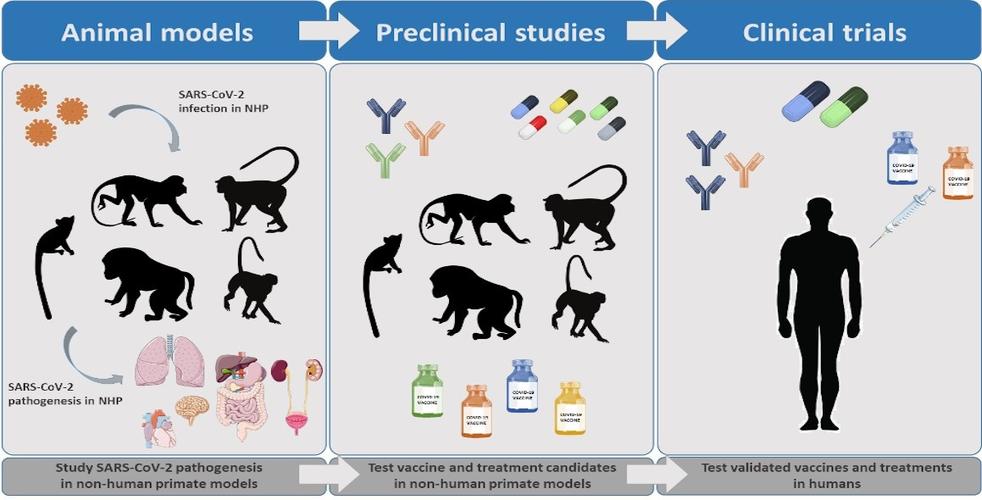
The Importance of Animal Studies
Animal studies are essential for several reasons. Firstly, they provide valuable insights into the drug’s behavior in a living organism, which is crucial for understanding its potential effects on humans. Secondly, they help identify the optimal dosage and treatment regimen for the drug. Lastly, animal studies help predict the drug’s potential side effects and toxicity, ensuring its safety for human use.
Types of Animals Used in Preclinical Cro
Various animals are used in preclinical cro, each offering unique advantages. Rodents, such as mice and rats, are the most commonly used animals due to their genetic similarity to humans. Other animals, such as rabbits, dogs, and monkeys, are also used depending on the drug’s intended use and target population.
Pharmacokinetic Studies
Pharmacokinetic studies focus on how the drug is absorbed, distributed, metabolized, and excreted in animals. These studies help determine the drug’s bioavailability, half-life, and elimination rate. By understanding these parameters, researchers can optimize the drug’s dosage and treatment regimen.
Pharmacodynamic Studies
Pharmacodynamic studies evaluate the drug’s effects on the body. These studies help determine the drug’s therapeutic index, efficacy, and potential side effects. By analyzing the drug’s pharmacodynamic properties, researchers can assess its potential for treating specific diseases.
Toxicity Studies
Toxicity studies are crucial in identifying the potential side effects and toxicity of a drug. These studies involve exposing animals to various doses of the drug and monitoring their physiological and behavioral responses. By evaluating the drug’s toxicity, researchers can ensure its safety for human use.
Table: Common Animals Used in Preclinical Cro
| Animal | Genetic Similarity to Humans | Common Uses |
|---|---|---|
| Mice | High | Pharmacokinetic and pharmacodynamic studies, toxicity testing |
| Rats | High | Pharmacokinetic and pharmacodynamic studies, toxicity testing |
| Rabbits | Medium | Toxicity testing, dermatological studies |
| Dogs | Low | Pharmacokinetic and pharmacodynamic studies, toxicity testing |
| Monkeys | High | Pharmacokinetic and pharmacodynamic studies, toxicity testing |
Challenges and Considerations
While animal studies are crucial in preclinical cro, they also come with challenges and considerations. One of the main challenges is the genetic and physiological differences between animals and humans. This can lead to discrepancies in drug response and efficacy. Additionally, ethical concerns arise regarding the use of animals in research. It is essential to ensure that animal studies are conducted with the utmost care and respect for the animals involved.
The Future of Animal Studies in Preclinical Cro
The field of animal studies in preclinical cro is continuously evolving. Advances in technology and research methods are making it possible to conduct more accurate and efficient studies. Additionally, alternative methods, such as in vitro and computational models, are being explored to reduce the reliance on animal testing. These advancements hold the promise of a more ethical and effective drug development process.
In conclusion, animal studies in preclinical cro are a critical component of the drug development process. By
