Understanding ADME and CRO Studies: A Comprehensive Guide for Researchers
When embarking on the journey of drug discovery and development, two critical aspects that often come into play are ADME (Absorption, Distribution, Metabolism, and Excretion) and CRO (Contract Research Organization) studies. These studies are pivotal in ensuring the safety, efficacy, and regulatory compliance of pharmaceuticals. In this detailed guide, we will delve into the intricacies of ADME and CRO studies, providing you with a comprehensive understanding of their significance and methodologies.
What is ADME?
ADME studies are a crucial component of drug development, focusing on how a drug behaves within the body. Let’s break down each aspect of ADME:
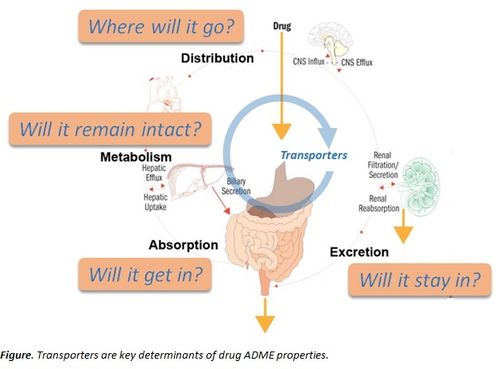
- Absorption: This refers to the process by which a drug enters the bloodstream from its site of administration. Factors such as the drug’s chemical properties, dosage form, and route of administration can influence absorption.
- Distribution: Once absorbed, the drug must travel to its target site. Distribution is influenced by factors like blood flow, tissue permeability, and protein binding.
- Metabolism: Metabolism involves the chemical transformation of the drug within the body. This process can either enhance or diminish the drug’s efficacy and toxicity.
- Excretion: Excretion is the elimination of the drug and its metabolites from the body. The primary routes of excretion are urine and feces, but other organs like the liver and kidneys also play a role.
ADME studies are essential for understanding the pharmacokinetics of a drug, which refers to the time course of drug absorption, distribution, metabolism, and excretion. This information is crucial for optimizing drug dosage, formulation, and administration.
Importance of ADME Studies
ADME studies are vital for several reasons:
- Drug Efficacy: Understanding how a drug behaves within the body helps in optimizing its dosage and formulation for maximum efficacy.
- Drug Safety: Identifying potential drug-drug interactions, toxicity, and metabolism pathways can help in predicting and mitigating adverse effects.
- Regulatory Compliance: ADME data is often required for regulatory approval, ensuring that the drug meets safety and efficacy standards.
Methods Used in ADME Studies
ADME studies employ various methodologies to assess drug behavior within the body. Some common techniques include:
- In Vitro Assays: These involve testing drug behavior in cells or tissues outside the body. Techniques like cell culture, enzyme assays, and receptor binding assays are commonly used.
- In Vivo Studies: These involve administering the drug to animals or humans to observe its behavior within the body. Techniques like pharmacokinetic studies, toxicology studies, and bioavailability studies are commonly used.
- Computational Modeling: This involves using mathematical models to predict drug behavior within the body. Techniques like pharmacokinetic-pharmacodynamic (PK/PD) modeling and physiologically-based pharmacokinetic (PBPK) modeling are commonly used.
What is a CRO?
A Contract Research Organization (CRO) is a company that provides research services to pharmaceutical, biotechnology, and medical device companies. CROs play a crucial role in drug development by offering expertise and resources that may not be available in-house.
Services Provided by CROs
CROs offer a wide range of services, including:
- Drug Discovery: CROs can assist in identifying and optimizing drug candidates through hit identification, lead optimization, and preclinical studies.
- Preclinical Development: CROs can conduct ADME, toxicology, and pharmacology studies to assess the safety and efficacy of drug candidates.
- Clinical Development: CROs can manage and conduct clinical trials, including patient recruitment, data collection, and analysis.
- Regulatory Affairs: CROs can assist with regulatory submissions, ensuring that the drug meets the necessary standards for approval.
Benefits of Working with a CRO
Collaborating with a CRO offers several benefits:
- Expertise: CRO
