Understanding Advanced Clinical Research: A Comprehensive Guide for Participants
Are you considering participating in an advanced clinical research study? If so, it’s essential to have a thorough understanding of what this entails. Advanced clinical research, often referred to as clinical trials, plays a crucial role in the development of new treatments and medications. In this article, we will delve into the various aspects of advanced clinical research, providing you with the knowledge needed to make an informed decision.
What is Advanced Clinical Research?
Advanced clinical research involves the testing of new medical treatments, drugs, or devices in human subjects. These studies are conducted to determine the safety, efficacy, and optimal use of these interventions. Participants in these studies are vital to the advancement of medical science and the improvement of healthcare outcomes.
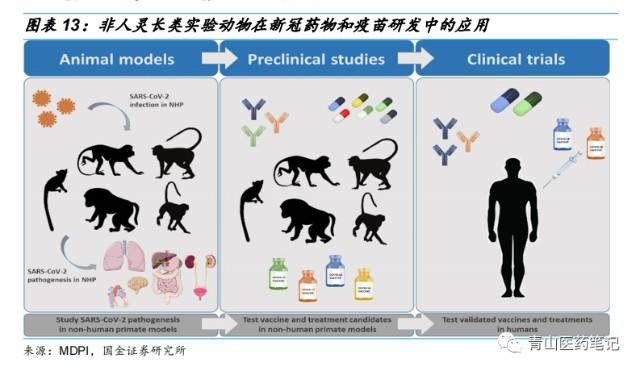
Types of Advanced Clinical Research Studies
There are several types of advanced clinical research studies, each with its unique characteristics:
| Type of Study | Description |
|---|---|
| Phase 1 | Initial testing of a new treatment in a small group of healthy volunteers to assess safety and dosage. |
| Phase 2 | Testing the new treatment in a larger group of patients to evaluate its effectiveness and side effects. |
| Phase 3 | Extensive testing of the new treatment in a large, diverse group of patients to confirm its effectiveness and monitor side effects. |
| Phase 4 | Post-marketing surveillance to monitor the long-term effects of the treatment in a broader population. |
Benefits of Participating in Advanced Clinical Research
Participating in an advanced clinical research study can offer several benefits:
-
Access to new treatments that may not be available to the general public.
-
Free or reduced-cost medical care during the study.
-
Contribution to the advancement of medical science and the improvement of healthcare outcomes.
-
Opportunity to receive additional attention from healthcare professionals.
Considerations Before Participating
Before deciding to participate in an advanced clinical research study, consider the following factors:
-
Understanding the study’s purpose, duration, and requirements.
-
Reviewing the potential risks and benefits associated with the study.
-
Assessing your eligibility for the study, including age, gender, medical history, and other criteria.
-
Understanding the compensation, if any, for participating in the study.
How to Find and Apply for Advanced Clinical Research Studies
There are several ways to find and apply for advanced clinical research studies:
-
Visit clinical trial websites, such as ClinicalTrials.gov, to search for studies in your area or related to your condition.
-
Consult with your healthcare provider for recommendations on suitable studies.
-
Join a clinical research registry, which can match you with studies that fit your criteria.
What to Expect During the Study
Once you have been accepted into an advanced clinical research study, here’s what you can expect:
-
Initial screening to determine your eligibility for the study.
-
Regular follow-up visits to monitor your health and the effects of the treatment.
-
Completion of questionnaires and other assessments to evaluate the treatment’s impact.
-
Access to additional medical care and support throughout the study.
Conclusion
Participating in advanced clinical research can be a rewarding experience, offering the chance to contribute to medical science and potentially improve your health. By understanding the various aspects of advanced clinical research, you can make an informed decision about whether to participate in a study. Always remember to consult with your healthcare provider and carefully review the study’s details before making a decision.
