Embarking on a journey through the vibrant landscape of China’s Contract Research Organization (CRO) sector, you’ll find a diverse array of companies offering a wide range of services to the pharmaceutical, biotech, and medical device industries. CRO China is not just a hub for research and development; it’s a testament to the nation’s growing influence in the global healthcare market.
Understanding the CRO Landscape
Let’s start by defining what a CRO is. A CRO is an organization that provides services to the pharmaceutical and biotech industries, such as clinical trials, data management, and regulatory affairs. In China, these companies play a crucial role in speeding up the drug development process, ensuring compliance with international standards, and reducing costs for pharmaceutical companies.
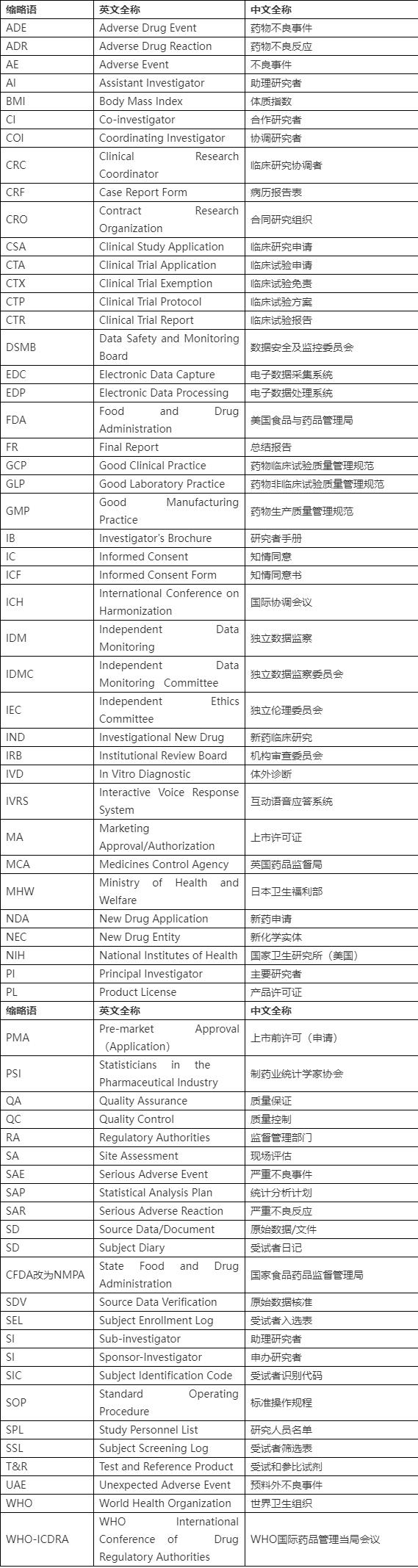
With the rise of innovative biopharmaceuticals and the increasing demand for high-quality research services, the CRO market in China has seen significant growth. According to a report by Grand View Research, the CRO market in China is expected to reach USD 18.5 billion by 2025, growing at a CAGR of 15.5% from 2019 to 2025.
Key Players in CRO China
Several key players have emerged as leaders in the CRO market in China. Here’s a brief overview of some of the most prominent companies:
| Company Name | Location | Specialization |
|---|---|---|
| Pharmaron | Shanghai | Drug discovery and development |
| WuXi AppTec | Shanghai | Research and development, manufacturing, and testing |
| Beijing PPD | Beijing | Clinical trials, data management, and regulatory affairs |
| Transcelerate Bio | Beijing | Biologics development and manufacturing |
These companies offer a comprehensive range of services, from early-stage research and development to late-stage clinical trials and regulatory submissions.
Services Offered by CROs in China
CROs in China provide a wide array of services to support drug development. Some of the key services include:

-
Clinical trials: CROs in China offer full-service clinical trial management, including site selection, patient recruitment, data collection, and analysis.
-
Data management: These companies provide data management services, including data entry, cleaning, and analysis.
-
Regulatory affairs: CROs in China assist with regulatory submissions, ensuring compliance with international and local regulations.
-
Biologics development: Many CROs in China specialize in the development of biologics, including cell therapy, gene therapy, and antibody-drug conjugates.
-
Manufacturing and testing: Some CROs in China offer manufacturing and testing services for pharmaceuticals and biologics.
Challenges and Opportunities
While the CRO market in China presents numerous opportunities, it also faces several challenges. One of the main challenges is the high cost of clinical trials, which can be a barrier for smaller pharmaceutical companies. Additionally, the regulatory environment in China can be complex, requiring companies to navigate a variety of regulations and standards.
Despite these challenges, the CRO market in China is expected to grow significantly in the coming years. The increasing demand for high-quality research services, coupled with the government’s focus on promoting the pharmaceutical industry, is expected to drive growth in the CRO sector.
Conclusion
CRO China is a dynamic and rapidly growing sector, offering a wide range of services to support drug development. With the right mix of expertise, experience, and resources, CROs in China are well-positioned to play a key role in the global healthcare market.
