Cro Clinical Data Management: A Comprehensive Guide
Clinical data management is a critical aspect of clinical research, and it plays a pivotal role in ensuring the integrity, quality, and accuracy of clinical trial data. As a participant in the clinical research process, it is essential to have a thorough understanding of cro clinical data management. This article aims to provide you with a detailed and multi-dimensional introduction to cro clinical data management, covering various aspects such as its importance, processes, tools, and challenges.
Understanding Cro Clinical Data Management
Cro clinical data management, also known as clinical research organization (CRO) data management, refers to the process of collecting, organizing, and analyzing clinical trial data. It involves various activities such as data entry, cleaning, validation, and reporting. The primary goal of cro clinical data management is to ensure that the data collected during clinical trials is accurate, complete, and compliant with regulatory requirements.
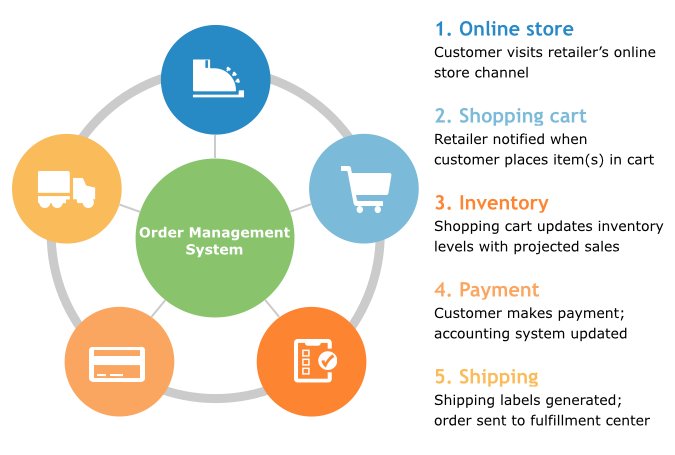
The Importance of Cro Clinical Data Management
Effective cro clinical data management is crucial for several reasons:
-
Ensuring Data Accuracy and Integrity: Accurate and reliable data is essential for making informed decisions during clinical trials. Cro clinical data management helps in maintaining the integrity of the data, reducing the risk of errors and biases.
-
Compliance with Regulatory Standards: Regulatory authorities, such as the FDA and EMA, have strict guidelines for clinical trial data management. Adhering to these standards is crucial for obtaining approval for new drug applications.
-
Optimizing Study Efficiency: Efficient data management can significantly reduce the time and cost associated with clinical trials. It helps in identifying potential issues early on, allowing for timely interventions.
-
Enhancing Data Quality: High-quality data leads to better-informed decisions and more reliable results. Cro clinical data management ensures that the data collected is of the highest standard.
Processes Involved in Cro Clinical Data Management
The cro clinical data management process typically involves the following stages:
-
Data Collection: This involves gathering data from various sources, such as electronic health records, patient diaries, and laboratory reports.
-
Data Entry: The collected data is entered into an electronic data capture (EDC) system, which is a software application designed for clinical data management.
-
Data Cleaning: This stage involves identifying and correcting errors, inconsistencies, and missing data in the entered data.
-
Data Validation: The cleaned data is validated to ensure its accuracy and completeness. This may involve checking for data outliers, inconsistencies, and adherence to regulatory guidelines.
-
Data Analysis: The validated data is analyzed to extract meaningful insights and conclusions.
-
Data Reporting: The final results are reported in the form of clinical study reports, regulatory submissions, and presentations.
Tools and Technologies Used in Cro Clinical Data Management
Several tools and technologies are used in cro clinical data management to streamline the process and improve data quality:
-
Electronic Data Capture (EDC): EDC systems are used for data entry, cleaning, and validation. They help in reducing errors and improving data accuracy.
-
Data Management Systems (DMS): DMS are used for managing and organizing clinical trial data. They provide a centralized repository for storing and accessing data.
-
Clinical Data Interchange Standards Consortium (CDISC): CDISC standards are used for organizing and structuring clinical trial data. They ensure consistency and interoperability across different systems.
-
Data Cleaning Tools: These tools help in identifying and correcting errors, inconsistencies, and missing data in the entered data.
-
Data Analysis Tools: These tools are used for analyzing and interpreting clinical trial data.
Challenges in Cro Clinical Data Management
Despite the advancements in technology and processes, cro clinical data management still faces several challenges:
-
Data Quality: Ensuring high-quality data remains a significant challenge. Errors, inconsistencies, and missing data can lead to inaccurate conclusions.
-
Data Security: Protecting sensitive patient data from unauthorized access and breaches is crucial. Compliance with data protection regulations is essential.
-
Data Integration: Integrating data from various sources and systems can be complex and time-consuming.
