Understanding the roles of CRO and CMO in the pharmaceutical industry is crucial for anyone involved in drug development or manufacturing. These two acronyms, CRO and CMO, represent specialized organizations that play pivotal roles in the lifecycle of a drug, from its inception to its final production and distribution. Let’s delve into what these organizations do and how they contribute to the pharmaceutical ecosystem.
What is a CRO?
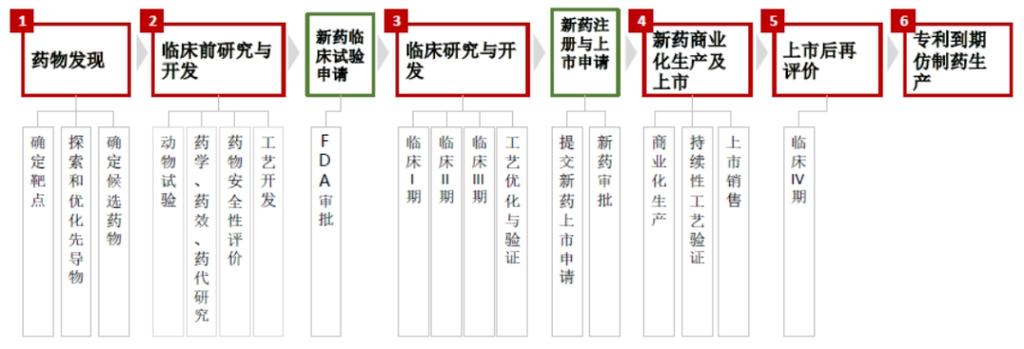
A CRO, or Contract Research Organization, is a company that provides specialized services to pharmaceutical and biotechnology companies. These services are focused on the research and development phase of drug discovery and development. CROs can handle a wide range of tasks, from early-stage research to late-phase clinical trials, and everything in between.
| Services Provided by CROs | Description |
|---|---|
| New Drug Discovery | Identifying potential drug candidates through various research methods. |
| Preclinical Studies | Testing drug candidates in the laboratory and on animals to assess their safety and efficacy. |
| Phase I, II, and III Clinical Trials | Testing drug candidates on human volunteers and patients to evaluate their safety, efficacy, and dosing regimens. |
| Regulatory Affairs | Assisting with the preparation and submission of regulatory documents to obtain approval for drug marketing. |
What is a CMO?
A CMO, or Contract Manufacturing Organization, is a company that provides pharmaceutical manufacturing services to pharmaceutical and biotechnology companies. CMOs can handle the production of active pharmaceutical ingredients (APIs), intermediates, and finished dosage forms, as well as packaging and labeling.
| Services Provided by CMOs | Description |
|---|---|
| API Production | Manufacturing the active ingredient of a drug. |
| Intermediates Production | Producing the chemical compounds that are used to make the active ingredient. |
| Finished Dosage Forms | Producing the final form of the drug, such as tablets, capsules, or injectables. |
| Packaging and Labeling | Assembling and labeling the finished dosage forms for distribution. |
While CROs and CMOs have distinct roles, they often work closely together in the drug development process. For example, a CRO might identify a promising new drug candidate and then collaborate with a CMO to produce the drug in bulk for clinical trials. This collaboration can continue through the drug’s lifecycle, with the CMO handling the production of the drug for commercial distribution once it is approved by regulatory authorities.
One of the key benefits of using CRO and CMO services is the ability to leverage the expertise and resources of these specialized organizations. Pharmaceutical companies can focus on their core competencies, such as drug discovery and development, while outsourcing the non-core activities to CRO and CMOs. This can lead to more efficient and cost-effective drug development and production processes.
Another important aspect of CRO and CMO services is the quality control and regulatory compliance they provide. These organizations are typically equipped with the necessary facilities, equipment, and expertise to ensure that the drugs they produce meet the highest quality standards and comply with regulatory requirements. This is crucial for the safety and efficacy of the drugs and for the trust of patients and healthcare providers.
In conclusion, CRO and CMO organizations are essential partners in the pharmaceutical industry. Their specialized services enable pharmaceutical companies to develop and produce new drugs more efficiently and effectively, while ensuring the quality and safety of the final product. As the pharmaceutical industry continues to evolve, the role of CRO and CMOs will likely become even more important in driving innovation and improving patient care.
